
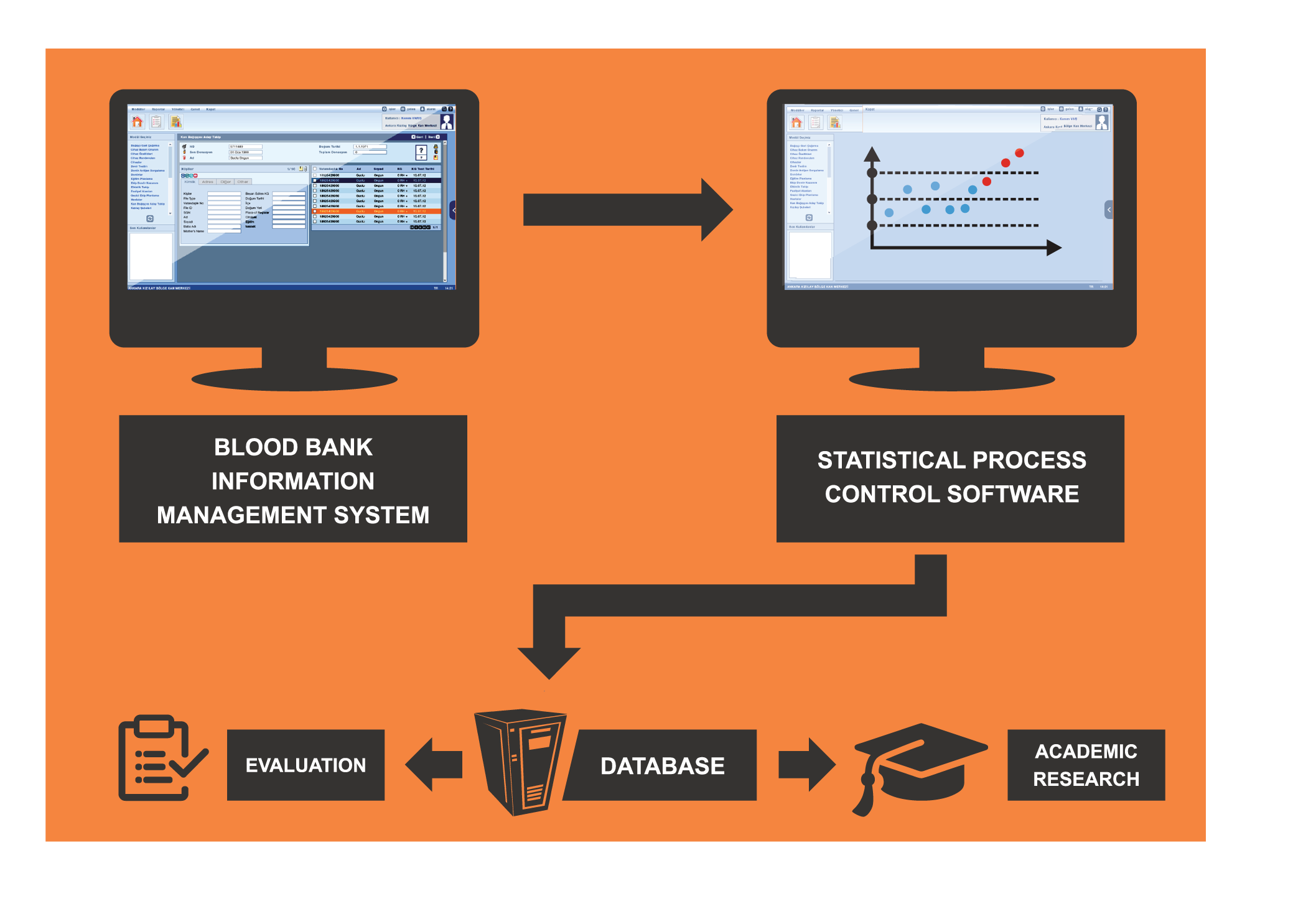
The system enables the management of quality processes followed in the blood establishments. In addition to the monitoring of external and internal quality control activities, the system allows for preparation, management and reporting of quality control documentation. The most recent versions of these documentations (SOP, quality control manual) can be viewed from the related modules of the Blood Bank Information Management System (HemOnline). The system also ensures management of internal and external quality audit processes, quality control meetings and other activities related to quality management. It is possible to retrieve data from HemOnline in order to see whether the quality control conditions set by the organization have been met. The system generates control graphics for key performance indicators over time with user defined thresholds and warns the user in cases of deviations. This ensures adoption of timely and effective preventive measures.
✔ SOP and Document Revision Monitoring Module
✔ Statistical Process
Control Module
✔ Product and Test Quality Control Module
- Product Quality Control
- Lab External Quality Control
✔ Device Calibration and Maintenance Planning
Module
✔ Sample Acceptance Module
✔ Management of Internal and
External Audit Processes
Please fill out the form to request a product brochure by e-mail.







